Framework for Kidney Health Follow-Up Among Neonates With Critical Cardiac Disease: A Report From the Neonatal Kidney Health Consensus Workshop
- PMID: 40079314
- PMCID: PMC12132693
- DOI: 10.1161/JAHA.124.040630
Framework for Kidney Health Follow-Up Among Neonates With Critical Cardiac Disease: A Report From the Neonatal Kidney Health Consensus Workshop
Abstract
Acute kidney injury is common among neonates with critical cardiac disease. Risk factors and associations with kidney-related outcomes are heterogeneous and distinct from other neonates. As survival of children with critical cardiac disease increases to adulthood, the burden of chronic kidney disease is increasing. Thirty percent to 50% of adults with congenital heart disease have impaired kidney function, even in the absence of prior kidney injury episodes. This may be related to the current standardized acute kidney injury criteria, which may not fully capture clinically meaningful kidney injury and long-term kidney health risks. An improved understanding of which neonates with critical cardiac disease should undergo kidney health follow-up is imperative. During the National Institutes of Health-supported Neonatal Kidney Health Consensus Workshop to Address Kidney Health meeting conducted in February 2024, a panel of 51 neonatal nephrology experts focused on at-risk groups: (1) preterm infants, (2) critically ill infants with acute kidney injury, and (3) infants with critical cardiac disease. The critical cardiac disease subgroup, comprising multidisciplinary experts, used a modified Delphi process to achieve consensus on recommendations for kidney health follow-up. In this report, we review available data on kidney health follow-up in critical cardiac disease and summarize the 2 consensus-based recommendations. We introduce novel diagnostic and risk-stratification tools for acute kidney injury diagnosis in neonates with cardiac disease to guide follow-up recommendations. Finally, we identify important knowledge gaps, representing areas of focus for future research. These should be prioritized to understand and improve long-term kidney health in critical cardiac disease.
Keywords: cardiac; chronic kidney; health; kidney; neonate; renal.
Conflict of interest statement
Drs Starr, Selewski, Harer, Ambalavanan, Slagle, Askenazi, Gist, Menon, Defrietas, and Charlton reported serving on the board of the Neonatal Kidney Collaborative. Dr Starr reporting receiving funding from the National Institutes of Health (NIH) and the Gerber Foundation outside the submitted work. Dr Harer reporting receiving funding from the NIH outside the submitted work. Dr Steflik reported receiving grants from Baxter outside the submitted work. Dr Ambalavanan reported receiving grants from the NIH during the conduct of the study as well as serving on the data and safety monitoring board for Oak Hill Bio and serving as medical advisor to ResBiotic/AlveolusBio outside the submitted work. Dr Fuhrman reporting receiving funding from the NIH outside the submitted work. Dr Kwiatkowski reported receiving grants from NIH during the conduct of the study. Dr Menon reported receiving grants from the Gerber Foundation outside the submitted work. Dr Rumple reporting receiving funding from the Arkansas Biosciences Institute, the National Center for Advancing Translational Sciences of the NIH, and the Marion B. Lyon New Scientist Development Award through the Arkansas Children's Research Institute outside the submitted work. Dr Sanderson reporting receiving funding from the NIH/National Institute on Diabetes and Digestive and Kidney Diseases (NIDDK) outside the submitted work. Dr Schuh reported receiving research funding from Otsuka and the NIH/NIDDK outside the submitted work. Dr Segar reported support for a study from Medtronics and funding from the NIH outside the submitted work. Dr Slagle reported receiving personal fees from Mozarc Medical and Bioporto outside the submitted work. Dr Soranno reported receiving funding from the NIH outside the submitted work. Dr Charlton reported receiving grants from the NIH and the NIDDK, being an investor in Zorro‐Flow, and consulting for Medtronics outside the submitted work. Dr Gist reported receiving consulting fees from Bioporto Diagnostics and Potrero Medical aswell as receiving grants from Gerber Foundation outside the submitted work. Dr Askenazi reported receiving personal fees from Nuwellis, Abbott, and Seastar; grants from Nuwellis, Bioporto, Leadiant, and Seastar; has a patent for Zorro‐Flow; and being the founder and chief scientific officer of Zorro‐Flow outside the submitted work. No other disclosures were reported.
Figures

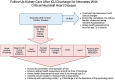
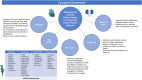
References
-
- Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, Gaies MG, Bertrandt RA, Smith AH, Reichle G, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med. 2021;49:e941–e951. doi: 10.1097/CCM.0000000000005165 - DOI - PubMed
-
- Blinder JJ, Asaro LA, Wypij D, Selewski DT, Agus MSD, Gaies M, Ferguson MA. Acute kidney injury after pediatric cardiac surgery: a secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med. 2017;18:638–646. doi: 10.1097/PCC.0000000000001185 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

