Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: a placebo-controlled double blind trial
- PMID: 33813110
- PMCID: PMC8015393
- DOI: 10.1016/j.cmi.2021.03.005
Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: a placebo-controlled double blind trial
Abstract
Objectives: To determine whether hydroxychloroquine decreases the risk of adverse outcome in patients with mild to moderate coronavirus disease 2019 (COVID-19) at high risk of worsening.
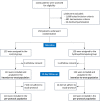
Methods: We conducted a multicentre randomized double-blind placebo-controlled trial evaluating hydroxychloroquine in COVID-19 patients with at least one of the following risk factors for worsening: need for supplemental oxygen, age ≥75 years, age between 60 and 74 years and presence of at least one co-morbidity. Severely ill patients requiring oxygen therapy >3 L/min or intensive care were excluded. Eligible patients were randomized in a 1:1 ratio to receive either 800 mg hydroxychloroquine on day 0 followed by 400 mg per day for 8 days or a placebo. The primary end point was a composite of death or start of invasive mechanical ventilation within 14 days following randomization. Secondary end points included mortality and clinical evolution at days 14 and 28, and viral shedding at days 5 and 10.
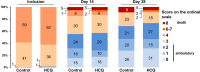
Results: The trial was stopped after 250 patients were included because of a slowing down of the pandemic in France. The intention-to-treat population comprised 123 and 124 patients in the placebo and hydroxychloroquine groups, respectively. The median age was 77 years (interquartile range 58-86 years) and 151/250 (60.4%) patients required oxygen therapy. The primary end point occurred in 9/124 (7.3%) patients in the hydroxychloroquine group and 8/123 (6.5%) patients in the placebo group (relative risk 1.12; 95% CI 0.45-2.80). The rates of positive SARS-CoV-2 RT-PCR tests at days 5 and 10 were 72.8% (75/103) and 57.1% (52/91) in the hydroxychloroquine group, versus 73.0% (73/100) and 56.6% (47/83) in the placebo group, respectively. No difference was observed between the two groups in any of the other secondary end points.
Conclusion: In this underpowered trial involving mainly older patients with mild to moderate COVID-19, patients treated with hydroxychloroquine did not experience better clinical or virological outcomes than those receiving the placebo.
Trial registration: ClinicalTrials.gov Identifier: NCT04325893 (https://clinicaltrials.gov/ct2/show/NCT04325893).
Keywords: Coronavirus disease 2019; Hydroxychloroquine; Placebo-controlled; Randomized controlled trial; Severe acute respiratory syndrome coronavirus 2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

