Clinical Presentation and Short- and Long-term Outcomes in Patients With Isolated Distal Deep Vein Thrombosis vs Proximal Deep Vein Thrombosis in the RIETE Registry
- PMID: 35830171
- PMCID: PMC9280612
- DOI: 10.1001/jamacardio.2022.1988
Clinical Presentation and Short- and Long-term Outcomes in Patients With Isolated Distal Deep Vein Thrombosis vs Proximal Deep Vein Thrombosis in the RIETE Registry
Abstract
Importance: Insufficient data exist about the clinical presentation, short-term, and long-term outcomes of patients with isolated distal deep vein thrombosis (IDDVT), that is, thrombosis in infrapopliteal veins without proximal extension or pulmonary embolism (PE).
Objective: To determine the clinical characteristics, short-term, and 1-year outcomes in patients with IDDVT and to compare the outcomes in unadjusted and multivariable adjusted analyses with patients who had proximal DVT.
Design, setting, and participants: This was a multicenter, international cohort study in participating sites of the Registro Informatizado Enfermedad Tromboembólica (RIETE) registry conducted from March 1, 2001, through February 28, 2021. Patients included in this study had IDDVT. Patients with proximal DVT were identified for comparison. Patients were excluded if they had a history of asymptomatic DVT, upper-extremity DVT, coexisting PE, or COVID-19 infection.
Main outcomes and measures: Primary outcomes were 90-day and 1-year mortality, 1-year major bleeding, and 1-year venous thromboembolism (VTE) deterioration, which was defined as subsequent development of proximal DVT or PE.
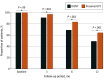
Results: A total of 33 897 patients were identified with isolated DVT (without concomitant PE); 5938 (17.5%) had IDDVT (mean [SD] age, 61 [17] years; 2975 male patients [50.1%]), and 27 959 (82.5%) had proximal DVT (mean [SD] age, 65 [18] years; 14 315 male patients [51.2%]). Compared with individuals with proximal DVT, those with IDDVT had a lower comorbidity burden but were more likely to have had recent surgery or to have received hormonal therapy. Patients with IDDVT had lower risk of 90-day mortality compared with those with proximal DVT (odds ratio [OR], 0.47; 95% CI, 0.40-0.55). Findings were similar in 1-year unadjusted analyses (hazard ratio [HR], 0.52; 95% CI, 0.46-0.59) and adjusted analyses (HR, 0.72; 95% CI, 0.64-0.82). Patients with IDDVT had a lower 1-year hazard of VTE deterioration (HR, 0.83; 95% CI, 0.69-0.99). In 1-year adjusted analyses of patients without an adverse event within the first 3 months, IDDVT was associated with lower risk of VTE deterioration (adjusted HR, 0.48; 95% CI, 0.24-0.97). By 1-year follow-up, symptoms or signs of postthrombotic syndrome were less common in patients with IDDVT (47.6% vs 60.5%).
Conclusions and relevance: Results of this cohort study suggest that patients with IDDVT had a less ominous prognosis compared with patients with proximal DVT. Such differences were likely multifactorial, including the differences in demographics, risk factors, comorbidities, particularly for all-cause mortality, and a potential association of thrombus location with VTE deterioration and postthrombotic syndrome. Randomized clinical trials are needed to assess the optimal long-term management of IDDVT.
Conflict of interest statement
Figures



References
-
- Galanaud JP, Sevestre-Pietri MA, Bosson JL, et al. ; OPTIMEV-SFMV Investigators . Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. 2009;102(3):493-500. doi:10.1160/TH09-01-0053 - DOI - PubMed
-
- Galanaud JP, Quenet S, Rivron-Guillot K, et al. ; RIETE Investigators . Comparison of the clinical history of symptomatic isolated distal deep vein thrombosis vs proximal deep vein thrombosis in 11 086 patients. J Thromb Haemost. 2009;7(12):2028-2034. doi:10.1111/j.1538-7836.2009.03629.x - DOI - PubMed

