Pharmacokinetics of Generic Pediatric Dolutegravir Dispersible Tablet in Thai Young Children Living With HIV Weighing Below Twenty Kilograms
- PMID: 39018516
- PMCID: PMC11250107
- DOI: 10.1097/INF.0000000000004366
Pharmacokinetics of Generic Pediatric Dolutegravir Dispersible Tablet in Thai Young Children Living With HIV Weighing Below Twenty Kilograms
Abstract
Introduction: Dolutegravir (DTG) dispersible tablet (DTG-DT) is a pediatric-friendly formulation. We aimed to describe the pharmacokinetics and virologic responses of generic DTG-DT in children weighing <20 kg.
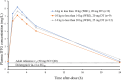
Methods: Children living with HIV-1 and <7 years of age weighing 6 to <20 kg were eligible. A generic 10-mg scored DTG-DT was administered to children using 3 weight bands (WB): WB1 (6 to <10 kg), WB2 (10 to <14 kg) and WB3 (14 to <20 kg), at doses of 20 mg (higher than World Health Organization recommendation of 15 mg), 20 mg and 25 mg, respectively. Steady-state intensive pharmacokinetics (PK) was performed in fasting condition with blood sampling at predose and 1, 2, 3, 4, 6 and 24 hours postdose. DTG PK parameters were estimated using a noncompartmental analysis, and DTG trough concentrations (C 24 ) and 24-hour area under the concentration-time curve were calculated. Comparisons were made with ODYSSEY and IMPAACT 2019. And 90% effective concentration of 0.32 mg/L was used as a reference individual DTG C 24 concentration.
Results: From August 2021 to March 2023, 29 Thai children with a median (interquartile range) age of 3.2 (1.5-4.8) years were enrolled; 8 in WB1, 9 in WB2 and 12 in WB3. All children were treatment experienced and 59% had HIV RNA <200 copies/mL. Overall geometric mean (coefficient of variation percentage) DTG C 24 was 1.0 (46%) mg/L [WB1, 0.9 (53%); WB2, 0.9 (27%); WB3, 1.2 (51%)]. Geometric mean (coefficient of variation percentage) 24-hour area under the concentration-time curve was 83.2 (24%) mg h/L [WB1, 84.3 (31%); WB2, 76.9 (16%); WB3, 87.6 (25%)]. At weeks 24 and 48, 90% and 92% of participants had plasma HIV RNA <200 copies/mL.
Conclusions: Generic DTG-DT provided adequate drug exposure in children weighing 6 to <20 kg. The exploratory dose of DTG 20 mg for children weighing 6 to <10 kg showed similar PK parameters to World Health Organization doses in the other WB.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- World Health Organization. The Global Health Observatory: Explore a World of Health Data: HIV. 2022. Available at: https://www.who.int/data/gho/data/themes/hiv-aids#:~:text=Globally%2C%20.... Accessed July 16, 2022.
-
- Clinton Health Access Initiative. Groundbreaking Agreement Reduces by 75% the Cost of HIV Treatment for Children in Low- and Middle-income Countries. 2022. Available at: https://www.clintonhealthaccess.org/groundbreaking-agreement-reduces-by-.... Accessed April 10, 2022.
-
- Min S, Sloan L, DeJesus E, et al. . Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737–1745. - PubMed
-
- UNICEF. Paediatric Care and Treatment. 2023. Available at: https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/. Accessed April 10, 2022.
-
- Ruxrungtham K, Chokephaibulkit K, Chetchotisakd P, et al. . Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2021/2022. Nonthaburi: Division of AIDS and STIs, Department of Disease Control; 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

