COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study
- PMID: 32593339
- PMCID: PMC7316447
- DOI: 10.1016/S2352-4642(20)30177-2
COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study
Abstract
Background: To date, few data on paediatric COVID-19 have been published, and most reports originate from China. This study aimed to capture key data on children and adolescents with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across Europe to inform physicians and health-care service planning during the ongoing pandemic.
Methods: This multicentre cohort study involved 82 participating health-care institutions across 25 European countries, using a well established research network-the Paediatric Tuberculosis Network European Trials Group (ptbnet)-that mainly comprises paediatric infectious diseases specialists and paediatric pulmonologists. We included all individuals aged 18 years or younger with confirmed SARS-CoV-2 infection, detected at any anatomical site by RT-PCR, between April 1 and April 24, 2020, during the initial peak of the European COVID-19 pandemic. We explored factors associated with need for intensive care unit (ICU) admission and initiation of drug treatment for COVID-19 using univariable analysis, and applied multivariable logistic regression with backwards stepwise analysis to further explore those factors significantly associated with ICU admission.
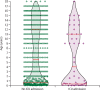
Findings: 582 individuals with PCR-confirmed SARS-CoV-2 infection were included, with a median age of 5·0 years (IQR 0·5-12·0) and a sex ratio of 1·15 males per female. 145 (25%) had pre-existing medical conditions. 363 (62%) individuals were admitted to hospital. 48 (8%) individuals required ICU admission, 25 (4%) mechanical ventilation (median duration 7 days, IQR 2-11, range 1-34), 19 (3%) inotropic support, and one (<1%) extracorporeal membrane oxygenation. Significant risk factors for requiring ICU admission in multivariable analyses were being younger than 1 month (odds ratio 5·06, 95% CI 1·72-14·87; p=0·0035), male sex (2·12, 1·06-4·21; p=0·033), pre-existing medical conditions (3·27, 1·67-6·42; p=0·0015), and presence of lower respiratory tract infection signs or symptoms at presentation (10·46, 5·16-21·23; p<0·0001). The most frequently used drug with antiviral activity was hydroxychloroquine (40 [7%] patients), followed by remdesivir (17 [3%] patients), lopinavir-ritonavir (six [1%] patients), and oseltamivir (three [1%] patients). Immunomodulatory medication used included corticosteroids (22 [4%] patients), intravenous immunoglobulin (seven [1%] patients), tocilizumab (four [1%] patients), anakinra (three [1%] patients), and siltuximab (one [<1%] patient). Four children died (case-fatality rate 0·69%, 95% CI 0·20-1·82); at study end, the remaining 578 were alive and only 25 (4%) were still symptomatic or requiring respiratory support.
Interpretation: COVID-19 is generally a mild disease in children, including infants. However, a small proportion develop severe disease requiring ICU admission and prolonged ventilation, although fatal outcome is overall rare. The data also reflect the current uncertainties regarding specific treatment options, highlighting that additional data on antiviral and immunomodulatory drugs are urgently needed.
Funding: ptbnet is supported by Deutsche Gesellschaft für Internationale Zusammenarbeit.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- WHO Novel coronavirus (2019-nCoV) situation report 5. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- WHO Novel coronavirus (2019-nCoV) situation report 148. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Zimmermann P, Goetzinger F, Ritz N. Severe and fatal COVID-19 occurs in young children. JAMA Pediatrics (in press).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

