Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial
- PMID: 35688164
- PMCID: PMC9173721
- DOI: 10.1016/S2213-2600(22)00180-1
Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial
Abstract
Background: Early intramuscular administration of SARS-CoV-2-neutralising monoclonal antibody combination, tixagevimab-cilgavimab, to non-hospitalised adults with mild to moderate COVID-19 has potential to prevent disease progression. We aimed to evaluate the safety and efficacy of tixagevimab-cilgavimab in preventing progression to severe COVID-19 or death.
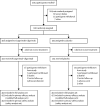
Methods: TACKLE is an ongoing, phase 3, randomised, double-blind, placebo-controlled study conducted at 95 sites in the USA, Latin America, Europe, and Japan. Eligible participants were non-hospitalised adults aged 18 years or older with a laboratory-confirmed SARS-CoV-2 infection (determined by RT-PCR or an antigen test) from any respiratory tract specimen collected 3 days or less before enrolment and who had not received a COVID-19 vaccination. A WHO Clinical Progression Scale score from more than 1 to less than 4 was required for inclusion and participants had to receive the study drug 7 days or less from self-reported onset of mild to moderate COVID-19 symptoms or measured fever. Participants were randomly assigned (1:1) to receive either a single tixagevimab-cilgavimab 600 mg dose (two consecutive 3 mL intramuscular injections, one each of 300 mg tixagevimab and 300 mg cilgavimab) or placebo. Randomisation was stratified (using central blocked randomisation with randomly varying block sizes) by time from symptom onset, and high-risk versus low-risk of progression to severe COVID-19. Participants, investigators, and sponsor staff involved in the treatment or clinical evaluation and monitoring of the participants were masked to treatment-group assignments. The primary endpoints were severe COVID-19 or death from any cause through to day 29, and safety. This study is registered with ClinicalTrials.gov, NCT04723394.
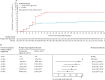
Findings: Between Jan 28, 2021, and July 22, 2021, 1014 participants were enrolled, of whom 910 were randomly assigned to a treatment group (456 to receive tixagevimab-cilgavimab and 454 to receive placebo). The mean age of participants was 46·1 years (SD 15·2). Severe COVID-19 or death occurred in 18 (4%) of 407 participants in the tixagevimab-cilgavimab group versus 37 (9%) of 415 participants in the placebo group (relative risk reduction 50·5% [95% CI 14·6-71·3]; p=0·0096). The absolute risk reduction was 4·5% (95% CI 1·1-8·0; p<0·0001). Adverse events occurred in 132 (29%) of 452 participants in the tixagevimab-cilgavimab group and 163 (36%) of 451 participants in the placebo group, and were mostly of mild or moderate severity. There were three COVID-19-reported deaths in the tixagevimab-cilgavimab group and six in the placebo group.
Interpretation: A single intramuscular tixagevimab-cilgavimab dose provided statistically and clinically significant protection against progression to severe COVID-19 or death versus placebo in unvaccinated individuals and safety was favourable. Treating mild to moderate COVID-19 earlier in the disease course with tixagevimab-cilgavimab might lead to more favourable outcomes.
Funding: AstraZeneca.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests HM has received consultation fees from AstraZeneca and is supported by the UK National Institute for Health Research's Comprehensive Biomedical Research Centre at University College London Hospitals. He has consulted for Millfield Medical Ltd on the development of a new continuous positive airway pressure machine. JASC reports serving on advisory boards for Pfizer and Eli Lilly; and serving on advisory boards and as a speaker for AstraZeneca and Roche. FDRH reports funding from AstraZeneca to cover meeting attendances and operationalisation of TACKLE in the UK as UK principal investigator. He has received funding by UK Research and Innovation and National Institute for Health and Care Research (NIHR) for national Urgent Public Health COVID-19 trials, and as Director of the NIHR Applied Research Collaboration, Oxford Thames Valley, and investigator on the Oxford Biomedical Research Centre and NIHR MedTech. FP has received personal fees and grants from Amgen, AstraZeneca, Boehringer Ingelheim, Ferrer, Kowa, Medix, Merck, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, Servier, and Silanes. KK has received research grants for the conduct of the TACKLE trial, reports funding from Regeneron, Eli Lilly, Merck, Pfizer, and Adagio, and serves as a speaker for Regeneron. DA, AT, SS, RHA, BHB, DB, PG, JJ, GCKWK, KWP, RMV, KS, VA, MNP, and MTE are employees of, and hold or may hold stock in, AstraZeneca.
Figures

Comment in
-
Early treatment to prevent progression of SARS-CoV-2 infection.Lancet Respir Med. 2022 Oct;10(10):930-931. doi: 10.1016/S2213-2600(22)00213-2. Epub 2022 Jun 7. Lancet Respir Med. 2022. PMID: 35688163 Free PMC article. No abstract available.
References
-
- Limb M. Covid-19: Scientists and medics warn that it is too soon to lift all restrictions in England. BMJ. 2022;376:o469. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

