Rationale and design of the CV-PREVITAL study: an Italian multiple cohort randomised controlled trial investigating innovative digital strategies in primary cardiovascular prevention
- PMID: 37451717
- PMCID: PMC10351259
- DOI: 10.1136/bmjopen-2023-072040
Rationale and design of the CV-PREVITAL study: an Italian multiple cohort randomised controlled trial investigating innovative digital strategies in primary cardiovascular prevention
Abstract
Introduction: Prevention of cardiovascular disease (CVD) is of key importance in reducing morbidity, disability and mortality worldwide. Observational studies suggest that digital health interventions can be an effective strategy to reduce cardiovascular (CV) risk. However, evidence from large randomised clinical trials is lacking.
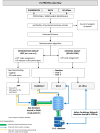
Methods and analysis: The CV-PREVITAL study is a multicentre, prospective, randomised, controlled, open-label interventional trial designed to compare the effectiveness of an educational and motivational mobile health (mHealth) intervention versus usual care in reducing CV risk. The intervention aims at improving diet, physical activity, sleep quality, psycho-behavioural aspects, as well as promoting smoking cessation and adherence to pharmacological treatment for CV risk factors. The trial aims to enrol approximately 80 000 subjects without overt CVDs referring to general practitioners' offices, community pharmacies or clinics of Scientific Institute for Research, Hospitalization and Health Care (Italian acronym IRCCS) affiliated with the Italian Cardiology Network. All participants are evaluated at baseline and after 12 months to assess the effectiveness of the intervention on short-term endpoints, namely improvement in CV risk score and reduction of major CV risk factors. Beyond the funded life of the study, a long-term (7 years) follow-up is also planned to assess the effectiveness of the intervention on the incidence of major adverse CV events. A series of ancillary studies designed to evaluate the effect of the mHealth intervention on additional risk biomarkers are also performed.
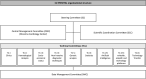
Ethics and dissemination: This study received ethics approval from the ethics committee of the coordinating centre (Monzino Cardiology Center; R1256/20-CCM 1319) and from all other relevant IRBs and ethics committees. Findings are disseminated through scientific meetings and peer-reviewed journals and via social media. Partners are informed about the study's course and findings through regular meetings.
Trial registration number: NCT05339841.
Keywords: Cardiac Epidemiology; Clinical trials; Health informatics; PREVENTIVE MEDICINE.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Authors and collaborators disclosed the relationships/activities/interests reported below. Grants or contracts from any entity: GSc, ACa, DPr, PW, ADiC, LV, LB, FCler, APa, LGM; Consulting fees: GSc, GL, SG, LV, LL, LGM, SL, IP; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: GSc, GA, ACa, DPr, GL, PW, SG, LV, LL, GPa, LB, LGi, EP, PA; Support for attending meetings and/or travel: DPr, LV, LL, EP, PA; Patents planned, issued or pending: LL, CGae; Participation on a Data Safety Monitoring Board or Advisory Board: GA, DPr, SG, LV, LL, LB, PA; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: GA, LV; Receipt of equipment, materials, drugs, medical writing, gifts or other services: LB, PA. Details are available in the individual ICMJE online disclosure forms (available upon request).
Figures
References
-
- European Commission . State of health in the EU – Italy, country health profile. 2021. Available: https://www.euro.who.int/__data/assets/pdf_file/0008/355985/Health-Profi...
-
- Healthcare expenditure Statistics: Eurostat (Hlth_Sha11_Hc). 2019. Available: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healt...
