Using pharmacokinetics for tailoring prophylaxis in people with hemophilia switching between clotting factor products: A scoping review
- PMID: 31294337
- PMCID: PMC6611373
- DOI: 10.1002/rth2.12204
Using pharmacokinetics for tailoring prophylaxis in people with hemophilia switching between clotting factor products: A scoping review
Erratum in
-
Corrigendum.Res Pract Thromb Haemost. 2019 Sep 16;3(4):771. doi: 10.1002/rth2.12258. eCollection 2019 Oct. Res Pract Thromb Haemost. 2019. PMID: 31624799 Free PMC article.
Abstract
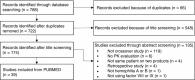
The objective of this scoping review is to summarize the current use of pharmacokinetics for tailoring prophylaxis in hemophilia patients switching between clotting factor products. Patients with hemophilia may require switching of clotting factor concentrates due to a variety of factors, but there have been perceived risks associated with switching, such as inhibitor development or suboptimal protection due to inadequate dosing while titrating treatment. Studies that look at patients switching from one clotting factor concentrate to another are categorized in terms of their primary and/or secondary objectives, notably biosimilarity and comparative pharmacokinetic studies and inhibitor development studies. Research on how best to switch concentrates with respect to dosing regimen are lacking, and currently a trial-and-error approach is used for dosing the new factor concentrate. In the future, studies looking at the predictability of pharmacokinetics (PK) of a new factor concentrate based on individual PK knowledge of the original factor concentrate may offer clinical benefit by providing a safer switching approach and protocol.
Keywords: drug substitution; factor IX; factor VIII; hemophilia A; hemophilia B.
Figures
References
-
- Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding, bleeding‐related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011:Cd003429. - PubMed
-
- Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor‐VIII‐deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. - PubMed
-
- Oldenburg J. Prophylaxis in bleeding disorders. Thromb Res. 2011;127(suppl 1):S14–7. - PubMed
-
- Berntorp E, Astermark J, Baghaei F, Bergqvist D, Holmstrom M, Ljungberg B, et al. Treatment of haemophilia A and B and von Willebrand's disease: summary and conclusions of a systematic review as part of a Swedish health‐technology assessment. Haemophilia. 2012;18:158–65. - PubMed
-
- Fischer K, Collins PW, Ozelo MC, Srivastava A, Young G, Blanchette VS. When and how to start prophylaxis in boys with severe hemophilia without inhibitors: communication from the SSC of the ISTH. J Thromb Haemost. 2016;14:1105–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources