The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation
- PMID: 9096408
- PMCID: PMC20384
- DOI: 10.1073/pnas.94.7.3414
The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation
Abstract
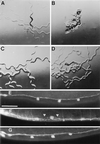
The gene unc-76 (unc, uncoordinated) is necessary for normal axonal bundling and elongation within axon bundles in the nematode Caenorhabditis elegans. The UNC-76 protein and two human homologs identified as expressed sequence tags are not similar to previously characterized proteins and thus represent a new protein family. At least one of these human homologs can function in C. elegans, suggesting that it, like UNC-76, acts in axonal outgrowth. We propose that the UNC-76 protein, which is found in cell bodies and processes of all neurons throughout development, either has a structural role in the formation and maintenance of axonal bundles or transduces signals to the intracellular machinery that regulates axonal extension and adhesion.
Figures




References
-
- Tessier-Lavigne M, Goodman C S. Science. 1996;274:1123–1133. - PubMed
-
- Hedgecock E M, Culotti J G, Thomson J N, Perkins L A. Dev Biol. 1985;111:158–170. - PubMed
-
- Desai C, Garriga G, McIntire S L, Horvitz H R. Nature (London) 1988;336:638–646. - PubMed
-
- McIntire S L, Garriga G, White J, Jacobson D, Horvitz H R. Neuron. 1992;8:307–322. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

