Characterization of serum amyloid A protein mRNA expression and secondary amyloidosis in the domestic duck
- PMID: 8962089
- PMCID: PMC26170
- DOI: 10.1073/pnas.93.25.14548
Characterization of serum amyloid A protein mRNA expression and secondary amyloidosis in the domestic duck
Abstract
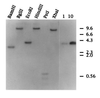
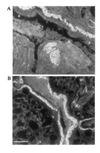
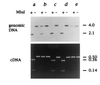
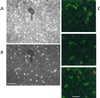
Secondary amyloidosis is a common disease of water fowl and is characterized by the deposition of extracellular fibrils of amyloid A (AA) protein in the liver and certain other organs. Neither the normal role of serum amyloid A (SAA), a major acute phase response protein, nor the causes of secondary amyloidosis are well understood. To investigate a possible genetic contribution to disease susceptibility, we cloned and sequenced SAA cDNA derived from livers of domestic ducks. This revealed that the three C-terminal amino acids of SAA are removed during conversion to insoluble AA fibrils. Analysis of SAA cDNA sequences from several animals identified a distinct genetic dimorphism that may be relevant to susceptibility to secondary amyloid disease. The duck genome contained a single copy of the SAA gene that was expressed in liver and lung tissue of ducklings, even in the absence of induction of acute phase response. Genetic analysis of heterozygotes indicated that only one SAA allele is expressed in livers of adult birds. Immunofluorescence staining of livers from adult ducks displaying early symptoms of amyloidosis revealed what appear to be amyloid deposits within hepatocytes that are expressing unusually high amounts of SAA protein. This observation suggests that intracellular deposition of AA may represent an early event during development of secondary amyloidosis in older birds.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

