A randomized controlled trial on the use of pessary plus progesterone to prevent preterm birth in women with short cervical length (P5 trial)
- PMID: 31775669
- PMCID: PMC6880495
- DOI: 10.1186/s12884-019-2513-2
A randomized controlled trial on the use of pessary plus progesterone to prevent preterm birth in women with short cervical length (P5 trial)
Abstract
Background: Preterm birth is the leading cause of mortality and disability in newborn and infants. Having a short cervix increases the risk of preterm birth, which can be accessed by a transvaginal ultrasound scan during the second trimester. In women with a short cervix, vaginal progesterone and pessary can both reduce this risk, which progesterone more established than cervical pessary. The aim of this study is to compare the use of vaginal progesterone alone versus the association of progesterone plus pessary to prevent preterm birth in women with a short cervix.
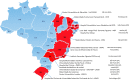
Methods: This is a pragmatic open-label randomized controlled trial that will take place in 17 health facilities in Brazil. Pregnant women will be screened for a short cervix with a transvaginal ultrasound between 18 0/7 until 22 6/7 weeks of gestational age. Women with a cervical length below or equal to 30 mm will be randomized to the combination of progesterone (200 mg) and pessary or progesterone (200 mg) alone until 36 + 0 weeks. The primary outcome will be a composite of neonatal adverse events, to be collected at 10 weeks after birth. The analysis will be by intention to treat. The sample size is 936 women, and a prespecified subgroup analysis is planned for cervical length (= < or > 25 mm). Categorical variables will be expressed as a percentage and continuous variables as mean with standard deviation. Time to delivery will be assessed with Kaplan-Meier analysis and Cox proportional hazard analysis.
Discussion: In clinical practice, the combination of progesterone and pessary is common however, few studies have studied this association. The combination of treatment might act in both the biochemical and mechanical routes related to the onset of preterm birth.
Trial registration: Brazilian Clinical Trial Registry (ReBec) RBR-3t8prz, UTN: U1111-1164-2636, 2014/11/18.
Keywords: Cervical length measurement; Pessaries; Progesterone; Randomized controlled trial.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- World Health Organization . Born too soon. Geneva: WHO Library; 2012.
-
- Tedesco RP, Passini R, Cecatti JG, Camargo RS, Pacagnella RC, Sousa MH. Estimation of preterm birth rate, associated factors and maternal morbidity from a demographic and health survey in Brazil. Matern Child Health J. 2013;17:1638–1647. - PubMed
-
- Hamilton BE, Martin JA, Osterman MJKS. Births: preliminary data for 2015. 2015. - PubMed
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous