Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals
- PMID: 9679147
- PMCID: PMC2133055
- DOI: 10.1083/jcb.142.2.499
Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals
Abstract
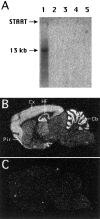
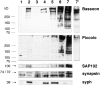
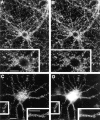
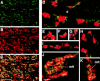
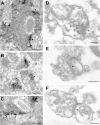
The molecular architecture of the cytomatrix of presynaptic nerve terminals is poorly understood. Here we show that Bassoon, a novel protein of >400,000 Mr, is a new component of the presynaptic cytoskeleton. The murine bassoon gene maps to chromosome 9F. A comparison with the corresponding rat cDNA identified 10 exons within its protein-coding region. The Bassoon protein is predicted to contain two double-zinc fingers, several coiled-coil domains, and a stretch of polyglutamines (24 and 11 residues in rat and mouse, respectively). In some human proteins, e.g., Huntingtin, abnormal amplification of such poly-glutamine regions causes late-onset neurodegeneration. Bassoon is highly enriched in synaptic protein preparations. In cultured hippocampal neurons, Bassoon colocalizes with the synaptic vesicle protein synaptophysin and Piccolo, a presynaptic cytomatrix component. At the ultrastructural level, Bassoon is detected in axon terminals of hippocampal neurons where it is highly concentrated in the vicinity of the active zone. Immunogold labeling of synaptosomes revealed that Bassoon is associated with material interspersed between clear synaptic vesicles, and biochemical studies suggest a tight association with cytoskeletal structures. These data indicate that Bassoon is a strong candidate to be involved in cytomatrix organization at the site of neurotransmitter release.
Figures


References
-
- Amaral, D.G., and M.P. Witter. 1994. Hippocampal Formation. In The Rat Nervous System. G. Paxinos, editor. Academic Press Limited, London, United Kingdom. 443–494.
-
- Burns ME, Augustine GJ. Synaptic structure and function: dynamic organization yields architectural precision. Cell. 1995;83:187–194. - PubMed
-
- Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69:214–223. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

