Towards personalized medicine: a scoping review of immunotherapy in sepsis
- PMID: 38807151
- PMCID: PMC11134696
- DOI: 10.1186/s13054-024-04964-6
Towards personalized medicine: a scoping review of immunotherapy in sepsis
Abstract
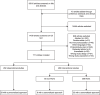
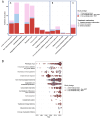
Despite significant progress in our understanding of the pathophysiology of sepsis and extensive clinical research, there are few proven therapies addressing the underlying immune dysregulation of this life-threatening condition. The aim of this scoping review is to describe the literature evaluating immunotherapy in adult patients with sepsis, emphasizing on methods providing a "personalized immunotherapy" approach, which was defined as the classification of patients into a distinct subgroup or subphenotype, in which a patient's immune profile is used to guide treatment. Subgroups are subsets of sepsis patients, based on any cut-off in a variable. Subphenotypes are subgroups that can be reliably discriminated from other subgroup based on data-driven assessments. Included studies were randomized controlled trials and cohort studies investigating immunomodulatory therapies in adults with sepsis. Studies were identified by searching PubMed, Embase, Cochrane CENTRAL and ClinicalTrials.gov, from the first paper available until January 29th, 2024. The search resulted in 15,853 studies. Title and abstract screening resulted in 1409 studies (9%), assessed for eligibility; 771 studies were included, of which 282 (37%) were observational and 489 (63%) interventional. Treatment groups included were treatments targeting the innate immune response, the complement system, coagulation and endothelial dysfunction, non-pharmalogical treatment, pleiotropic drugs, immunonutrition, concomitant treatments, Traditional Chinese Medicine, immunostimulatory cytokines and growth factors, intravenous immunoglobulins, mesenchymal stem cells and immune-checkpoint inhibitors. A personalized approach was incorporated in 70 studies (9%). Enrichment was applied using cut-offs in temperature, laboratory, biomarker or genetic variables. Trials often showed conflicting results, possibly due to the lack of patient stratification or the potential influence of severity and timing on immunomodulatory therapy results. When a personalized approach was applied, trends of clinical benefit for several interventions emerged, which hold promise for future clinical trials using personalized immunotherapy.
Keywords: Enrichment; Immunomodulation; Immunotherapy; Personalized; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
SJWO has received travel support from Fisher & Paykel Healthcare. MGN is a scientific founder of TTxD, Lemba and Biotrip. PP has received travel reimbursements and consulting fees from AM-Pharma, Adrenomed, EBI Paion, Sphingotec, and 4Teen4. EJG-B has received honoraria from Abbott Products Operations AG, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi and Xbiotech Inc; independent educational grants from Abbott Products Operations, bioMérieux Inc, InflaRx GmbH, Johnson & Johnson, MSD, UCB, Swedish Orphan Biovitrum AB; and funding from the Horizon 2020 European Grants ImmunoSep and RISCinCOVID and the Horizon Health grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). WJW reports a project grants from EU, Amsterdam UMC and NOW/ZonMW and receives ad hoc consulting fees from GSK, Pfizer, Sobi, AstraZeneca paid to the institution. APJV receives consultant fees from InflaRx paid to the institution. LAvV was supported by the
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

