Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial
- PMID: 25453443
- PMCID: PMC4322188
- DOI: 10.1016/S0140-6736(14)61184-3
Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial
Abstract
Background: Stenting is an alternative to endarterectomy for treatment of carotid artery stenosis, but long-term efficacy is uncertain. We report long-term data from the randomised International Carotid Stenting Study comparison of these treatments.
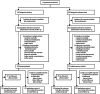
Methods: Patients with symptomatic carotid stenosis were randomly assigned 1:1 to open treatment with stenting or endarterectomy at 50 centres worldwide. Randomisation was computer generated centrally and allocated by telephone call or fax. Major outcomes were assessed by an independent endpoint committee unaware of treatment assignment. The primary endpoint was fatal or disabling stroke in any territory after randomisation to the end of follow-up. Analysis was by intention to treat ([ITT] all patients) and per protocol from 31 days after treatment (all patients in whom assigned treatment was completed). Functional ability was rated with the modified Rankin scale. This study is registered, number ISRCTN25337470.
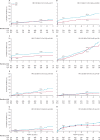
Findings: 1713 patients were assigned to stenting (n=855) or endarterectomy (n=858) and followed up for a median of 4·2 years (IQR 3·0-5·2, maximum 10·0). Three patients withdrew immediately and, therefore, the ITT population comprised 1710 patients. The number of fatal or disabling strokes (52 vs 49) and cumulative 5-year risk did not differ significantly between the stenting and endarterectomy groups (6·4% vs 6·5%; hazard ratio [HR] 1·06, 95% CI 0·72-1·57, p=0·77). Any stroke was more frequent in the stenting group than in the endarterectomy group (119 vs 72 events; ITT population, 5-year cumulative risk 15·2% vs 9·4%, HR 1·71, 95% CI 1·28-2·30, p<0·001; per-protocol population, 5-year cumulative risk 8·9% vs 5·8%, 1·53, 1·02-2·31, p=0·04), but were mainly non-disabling strokes. The distribution of modified Rankin scale scores at 1 year, 5 years, or final follow-up did not differ significantly between treatment groups.
Interpretation: Long-term functional outcome and risk of fatal or disabling stroke are similar for stenting and endarterectomy for symptomatic carotid stenosis.
Funding: Medical Research Council, Stroke Association, Sanofi-Synthélabo, European Union.
Copyright © 2015 Bonati et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Atherosclerosis: Carotid artery stenting versus endarterectomy--no difference in long-term outcomes.Nat Rev Cardiol. 2014 Dec;11(12):685. doi: 10.1038/nrcardio.2014.174. Epub 2014 Nov 4. Nat Rev Cardiol. 2014. PMID: 25367651 No abstract available.
-
The struggle of carotid artery stenting.Lancet. 2015 Feb 7;385(9967):490-1. doi: 10.1016/S0140-6736(14)61829-8. Epub 2014 Oct 14. Lancet. 2015. PMID: 25453444 No abstract available.
References
-
- North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. - PubMed
-
- European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. - PubMed
-
- Mas JL, Chatellier G, Beyssen B. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. - PubMed
-
- The SPACE Collaborative Group 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

